Or the 5 Types of Knowledge needed for successful Medical Device development
Developing innovative new products is hard but developing innovative new medical devices is even harder.
That’s because while both involve a great deal of knowledge, medical device development requires even more knowledge and across a broader range of domains. Some engineers assume a “broad” range of domains is simply having a team with both hardware and software engineers. Unfortunately not. The knowledge I’m talking about is much broader.
To emphasize the broad range of domain expertise required, I like to describe medical device development with the “Hand of Knowledge.” Teams will need knowledge of all 5 domains for successful medical device development.
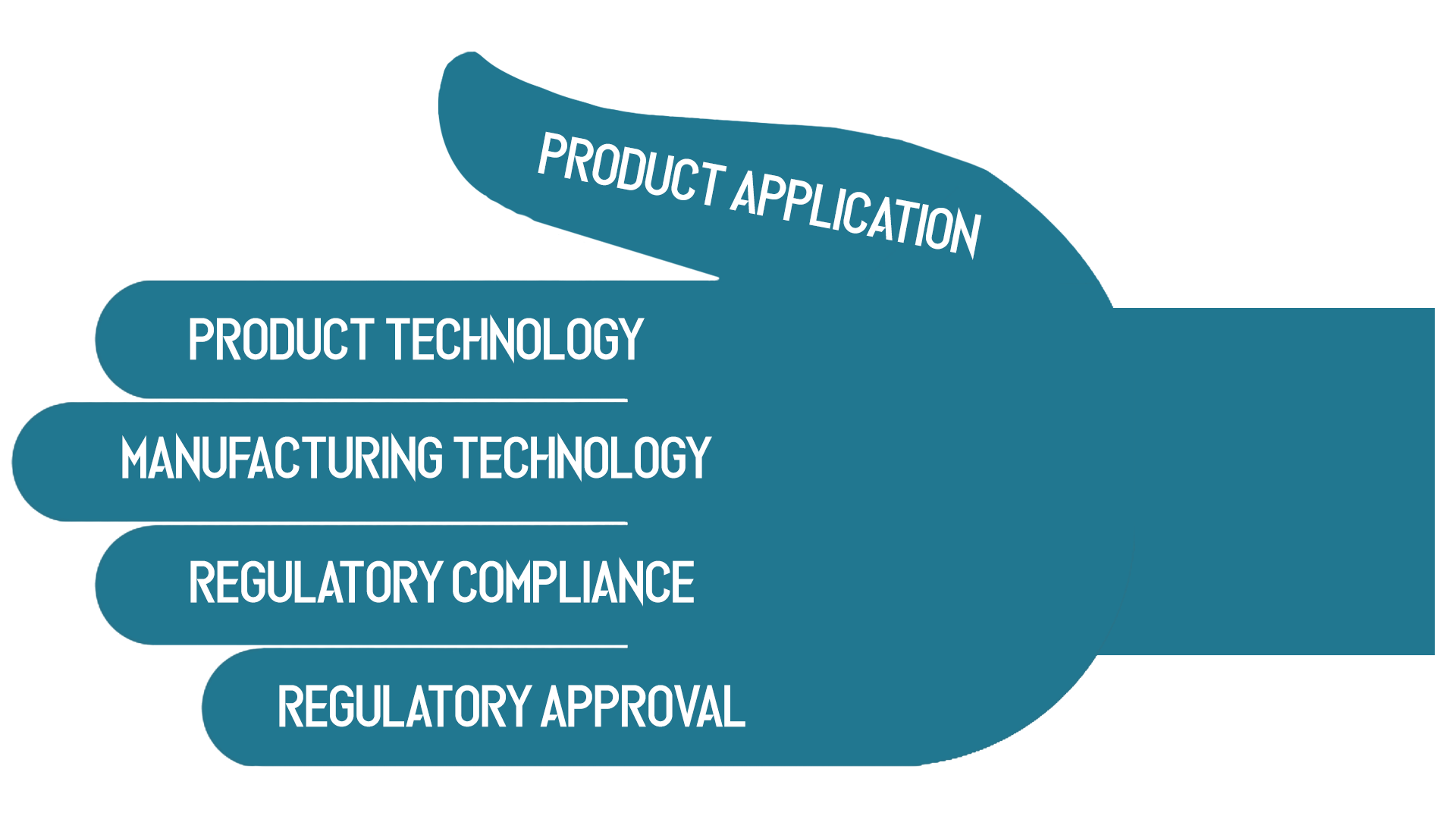
In other words, successful development of a new medical device demands deep knowledge of:
- Product Application – understanding the clinical environment where the new product will be used and the needs of clinicians and patients
- Product Technology – design of hardware, software, and packaging
- Manufacturing Technology – knowing how to reliably build the product and knowing supplier capabilities
- Regulatory Approval – understanding the approval process and key concerns of regulators
- Regulatory Compliance – understanding the requirements of all applicable regulations and standards
These last two categories of knowledge make medical device development especially challenging and a source of great confusion and frustration for people new to the medical device industry. That’s unavoidable since we work in a heavily regulated industry. But there can also be confusion among experienced medical device professionals about the first category of knowledge, Product Application. I’m surprised at how many product development teams I’ve worked with over the years that don’t truly understand how their new product is intended to be used. Or even why it needs to be developed. Given that situation, it’s very unlikely the new medical device will be a successful commercial product.
So maybe you’re thinking now, “How can anyone be deeply knowledgeable in all of these areas?” Well, the answer is no one person is (at least no one I’ve ever met) but a good cross-functional team can be. And here “cross-functional” can mean many different specialties: design engineers for mechanical, electrical, optical, software, and packaging; manufacturing engineers, clinicians, regulatory experts, material scientists, biochemists, etc. And even the combined expertise of all of these specialists may not be sufficient–if your new medical device is truly innovative then by definition your team is creating new knowledge as you progress in your development (and new knowledge is not something you can find in a textbook or article).
What does this mean for managing new product development? Well, first of all, your development team’s number one job is learning–studying existing knowledge, identifying knowledge gaps, and filling those gaps as quickly as possible. This might involve observations of doctors and nurses in a hospital, usability testing of prototypes with representative users, discussions with regulators of a proposed pathway, or performance testing of a key component technology. For engineers, it’s natural for us to focus first on all the cool technology we’re going to design into the new medical device. But that technology will be wasted if we don’t fully understand the users’ needs and how the product will be used (#1). And further, it won’t become a commercial product if we don’t understand efficient, reliable ways to manufacture it (#3) or what test data will be needed for regulatory approval (#4) or what procedures we need to follow during development (#5).
This emphasis of knowledge upfront leads naturally to viewing medical device development in two main parts–a learning dominant part followed by a task dominant part. This requires the product development organization to adopt two different mindsets for these two parts of development. For more information on this perspective see Two Mindsets for Successful Product Development and related links for agile-lean product development.
Bottom line: You need all five fingers to get a strong grip on medical device development.