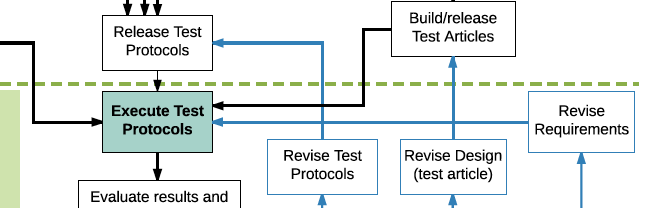
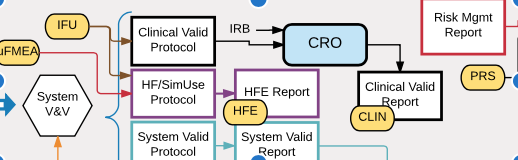
Newsletter V. 2019 Issue 2
Have employees new to medical devices? I offer practical training on Medical Device Design Controls either on site or at a public workshop. Agile Design Controls This is a three part recorded webinar series that illustrates a flexible and rigorous approach to managing design controls for software-intensive medical devices. I teamed up with software experts […]
Newsletter V. 2019 Issue 2 Read More »