V&V Flowchart by Aaron Joseph
Design verification and validation (V&V) is a key part of medical device development but can be confusing to engineers, especially those who are new to the medical device industry. This “V&V Flowchart” is intended to help clear up that confusion.
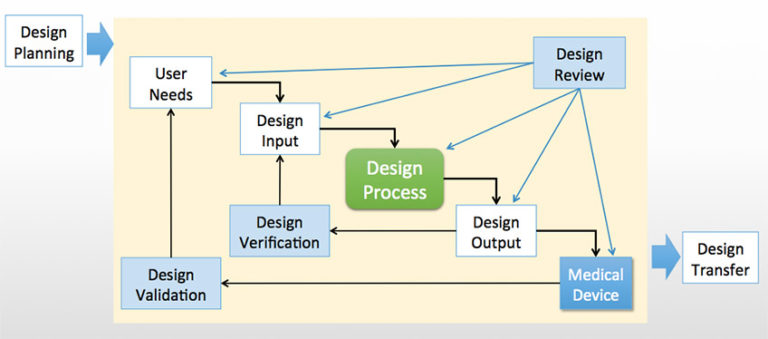
V&V testing is typically illustrated in the quality system with this classic design controls diagram, which gives a 20,000 foot view of the processes. Design verification shows that design outputs conform to design inputs (answers the question “Did we make the device right?”). Design validation shows that the product meets user needs (answers the bigger question “Did we make the right device?”). But this is not sufficient to understand how these processes work in a typical medical device company.
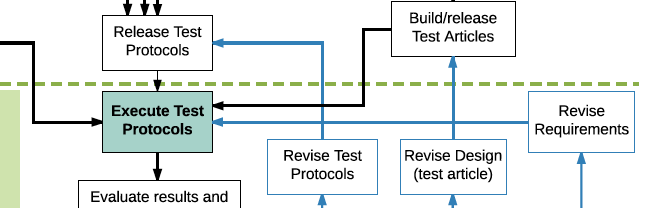
This V&V Flowchart provides a closer look at the basic steps in V&V testing and shows the overall sequence to complete V&V for a new product (a 5,000 foot view). The flowchart is based on a model where a single master V&V test plan organizes and controls all the V&V activities (sub-plans can be created underneath it for particularly complex areas of testing such as software). The test plan contains one or more test protocols and each protocol contains one or more test cases. The entire process is completed and documented with release of a V&V Summary Report. Note that some quality systems may use different terminology and different structures for managing V&V.

(Click here to download the flowchart as a pdf)
The flowchart emphasizes dependencies on different activities (part of the complexity of V&V testing) and in particular all the preparatory work needed before executing the tests–everything above the dotted line. There is a long list of prerequisites that need to be completed before doing the formal testing (the “run for record”). Rushing this preparatory work is tempting given tight product development schedules but that generally results in taking longer to finish V&V and will leave multiple compliance issues in the resulting documentation (translation: future audit findings and multiple CAPAs).
The flowchart also describes the basic workflow if there is a failure during testing or some other unacceptable test result (outlined in blue). There are 3 options/pathways following a test failure:
- The failure was caused by an error in the test instructions: revise the test protocol, re-release it, and repeat the failed test (alternatively, some quality systems will allow, under certain circumstances, to just redline the error in the protocol and mark the result as a Pass).
- The failure was caused by a flaw in the product: revise the design, build and release new test articles, and repeat the failed test. This can be very expensive, which is why you should be doing informal (engineering) testing before the formal V&V testing.
- The failure was caused by an incorrect design requirement: revise the requirement, re-release the requirement document, and repeat the failed test.
Medical device standards, such as the 60601 family of standards, play a large role in V&V testing (make certain you are familiar with all of the applicable standards for your product before starting V&V). For those tests, the requirements and test methods are already defined in the standard and test equipment and tools are usually managed by a test lab (e.g., electrical safety and EMC testing).
There can be many types of activities needed in V&V for a particular device. Design validation involving human testing (clinical evaluation, usability testing, etc.) will require additional steps not shown here.
Key Points:
- Formal V&V testing is not performed to discover something about the product (that testing should have been completed much earlier); the primary purpose is to demonstrate that the design is sound (by providing unambiguous objective evidence).
- Multiple dependencies in V&V testing require close coordination of activities across the product development organization
- V&V testing is process-intensive (and documentation intensive); proper planning and organization at the beginning will save considerable time in completing V&V
Originally posted on 12/12/17 at http://consensiainc.com/2017/12/12/vv-flowchart/

Pingback: Newsletter V. 2019 Issue 2 - Sunstone Pilot, Inc.