The Big Picture for Medical Device Risk Management
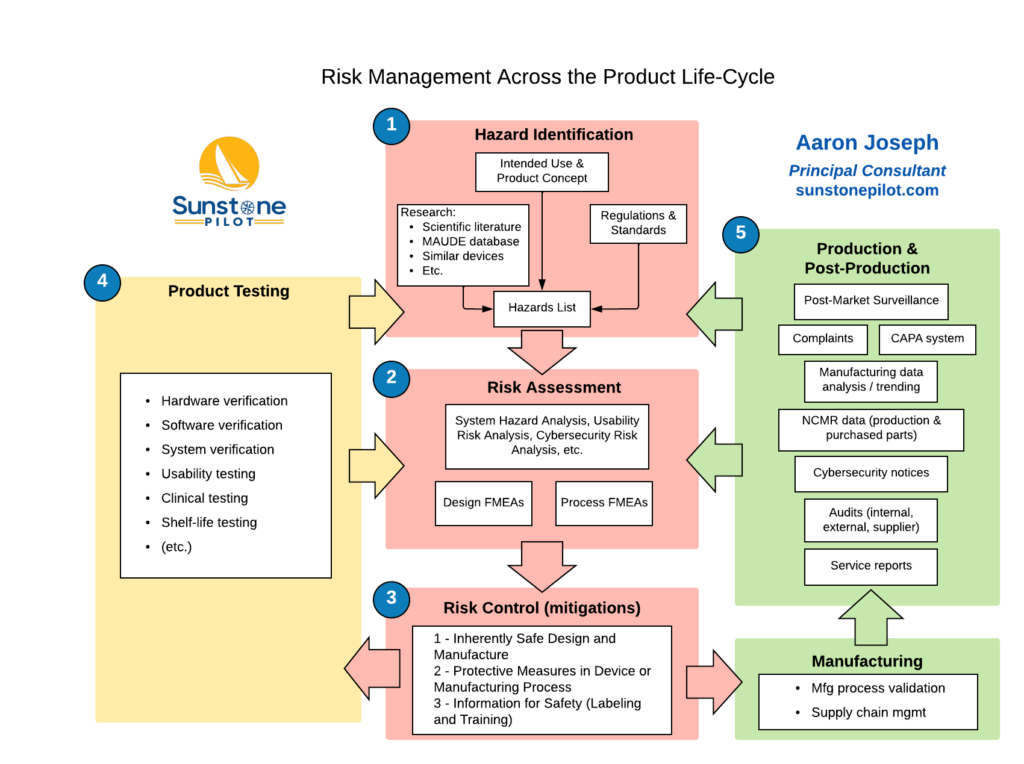
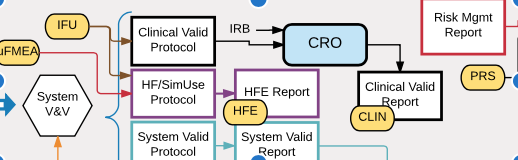
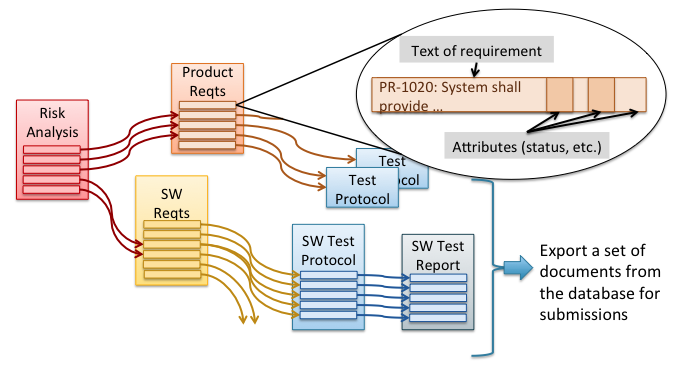
Understanding all the details of risk analysis methods and compliance with the ISO 14971 risk management standard can be quite complicated but fundamentally, for development of a new medical device, risk management is about answering these seven key questions: What are all the possible things that could go wrong? What are we most worried about? […]
The Big Picture for Medical Device Risk Management Read More »