What do design controls mean for medical device development?
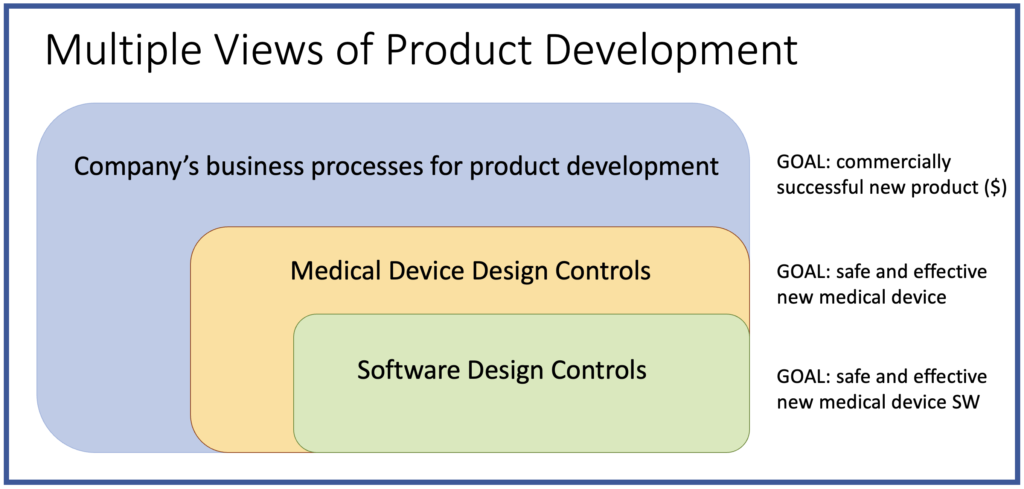
I regularly encounter misconceptions about what design controls are, with people overestimating them or underestimating them. I created this simple diagram to help provide a proper perspective on the role of design controls in medical device development. The diagram shows that design controls are a subset of a larger set of business processes the company uses to develop new products.
Underestimating design controls
Therefore, design controls are not a separate set of activities run in parallel to product development–they are a central part of development. This is the misconception of underestimating design controls, treating them as a documentation exercise, perhaps done by people at the other end of the building. This approach typically leads to many audit failures (and of course, entirely misses the benefits of design controls during development).
Overestimating design controls
I also find the opposite misconception where a company’s entire product development process is their design controls (overestimating design controls). Compliance with design controls alone is not enough to ensure successful new products. Design controls are focused on making sure new medical devices are safe and effective, but not necessarily profitable. Companies need a well-designed product development process that maximizes the chances of success for all of their product teams. Then appropriate design control procedures should be integrated into that overall process.
Software design controls
Software design controls are a further subset of the overall design controls. This is important to keep in mind for hardware-software medical devices where the software development needs to integrate with hardware and system development. For software-only medical devices (SaMD) the key point here is that fully satisfying software design controls (i.e., IEC 62304) is not sufficient alone for compliance. The product team also needs to satisfy the overall, system-level design controls. In other words, SaMD products are medical devices subject to general medical device design controls as well as software design controls.
For more information see: What Are Medical Device Design Controls?