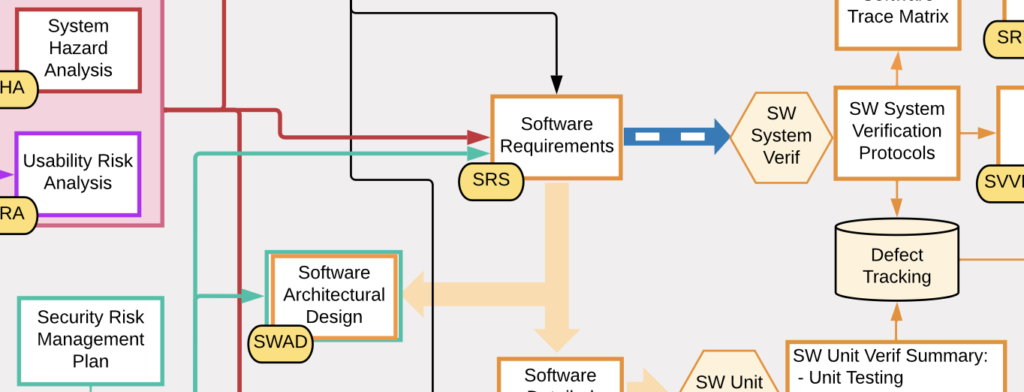
Rapid Compliance with the IEC 62304 Medical Software Standard
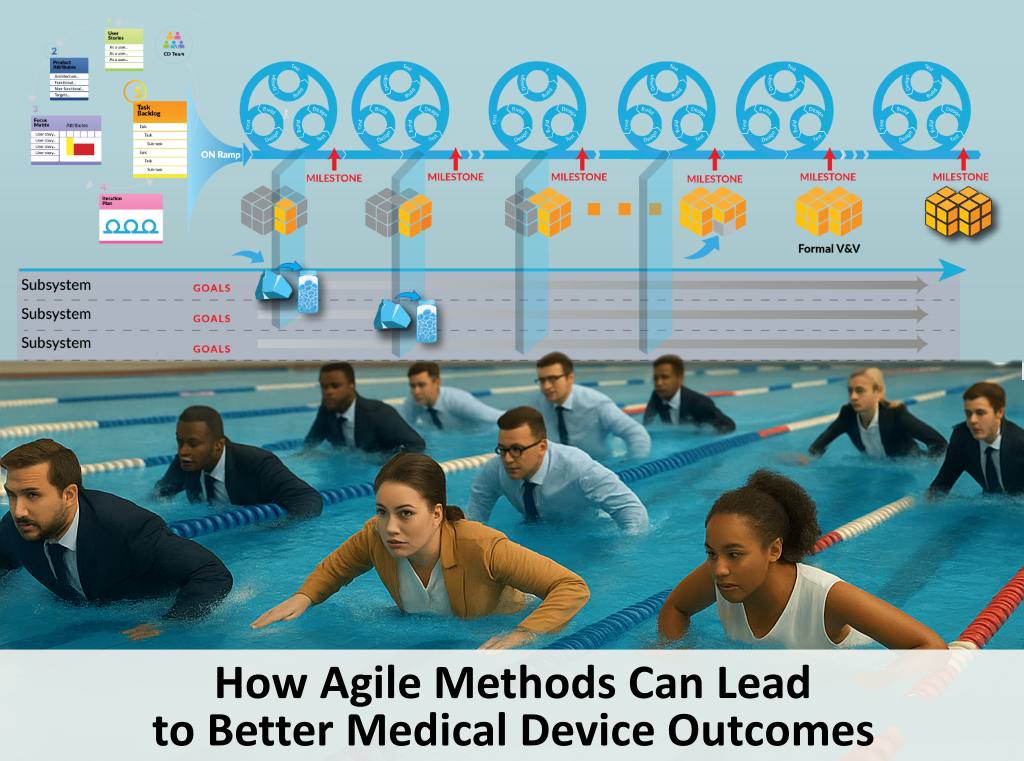
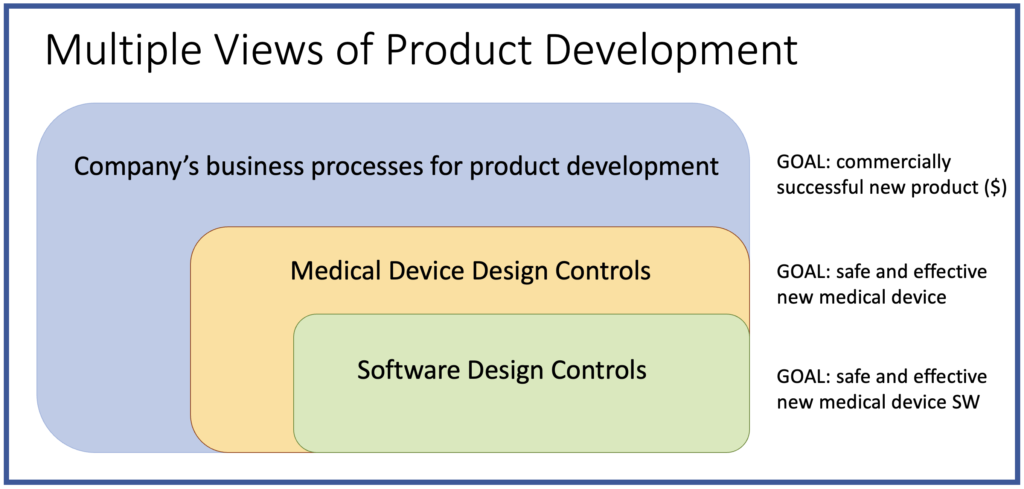
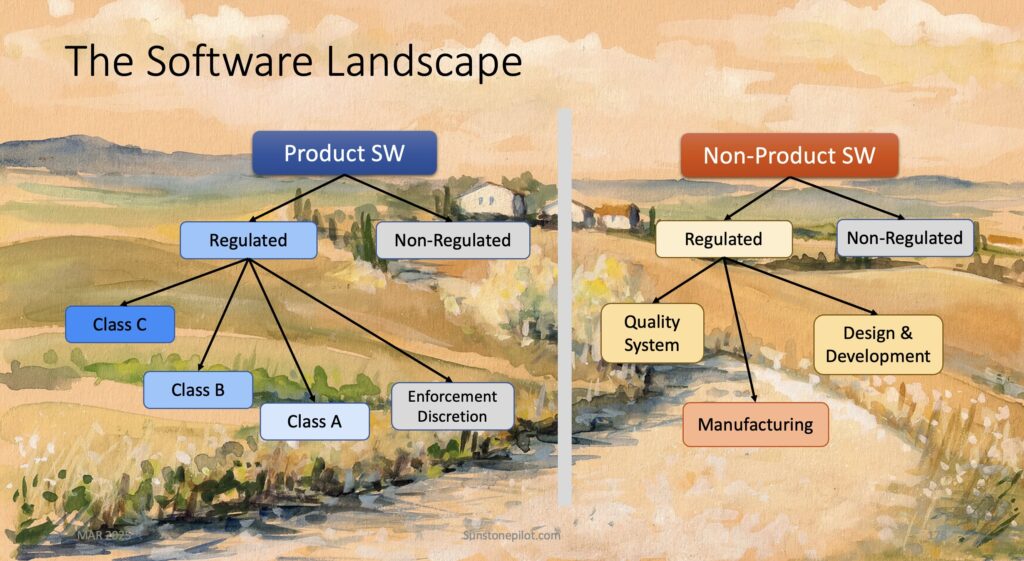
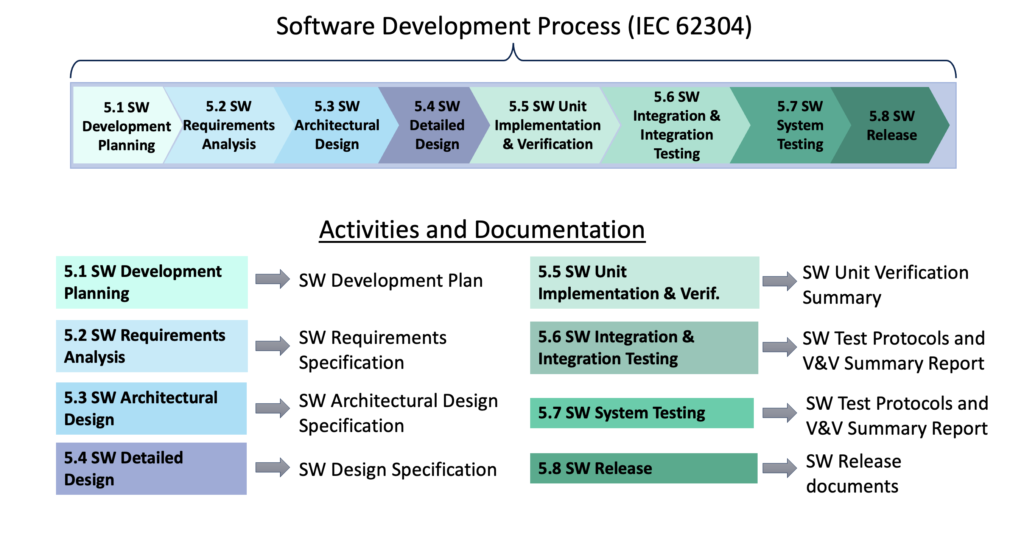
CLIENT Small Medical Device Manufacturer (<200 employees) Company Stage: Two hardware products launched; working on their first digital health product (SaMD) CHALLENGES Client had no experience with mobile or cloud software; relied 100% on development partner Software development partner had no experience with medical devices and regulations; they were making undocumented and uncontrolled changes to […]
Rapid Compliance with the IEC 62304 Medical Software Standard Read More »