V&V Flowchart
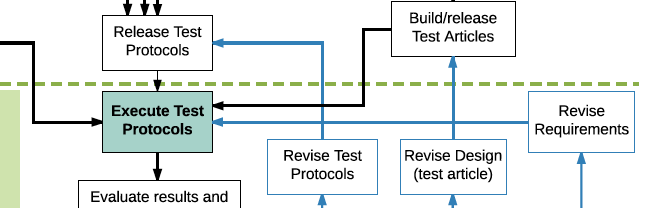
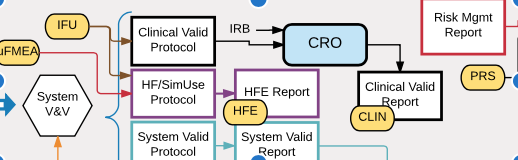
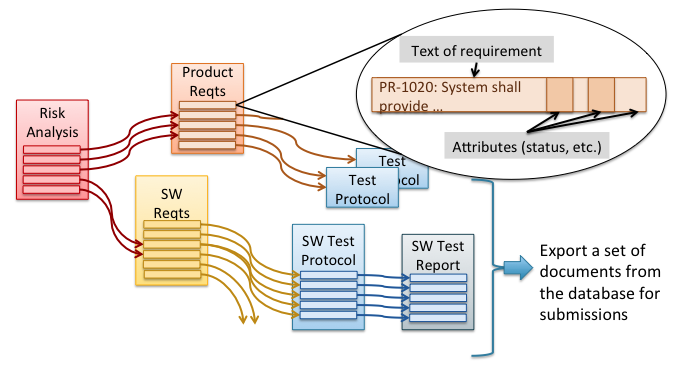
V&V Flowchart by Aaron Joseph Design verification and validation (V&V) is a key part of medical device development but can be confusing to engineers, especially those who are new to the medical device industry. This “V&V Flowchart” is intended to help clear up that confusion. V&V testing is typically illustrated in the quality system with this […]