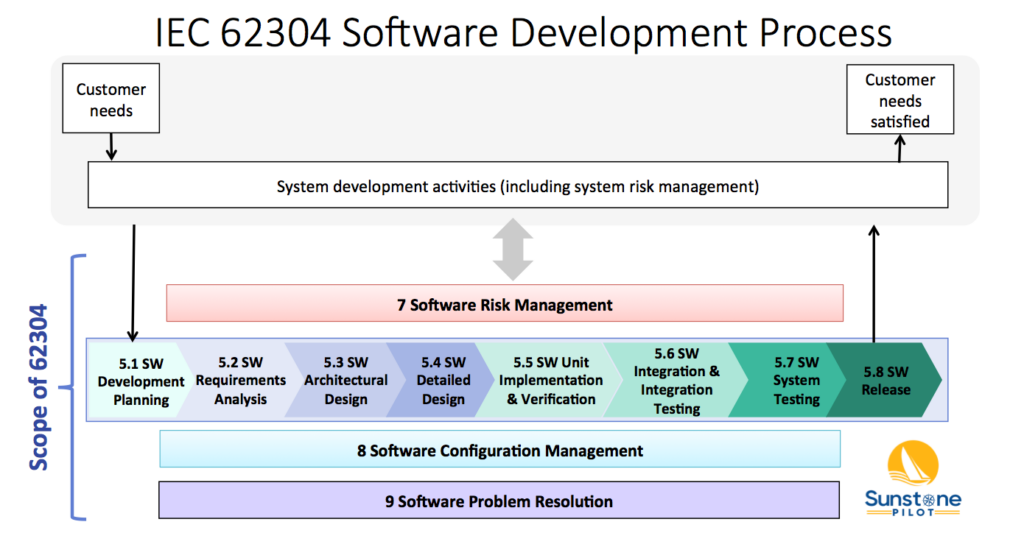
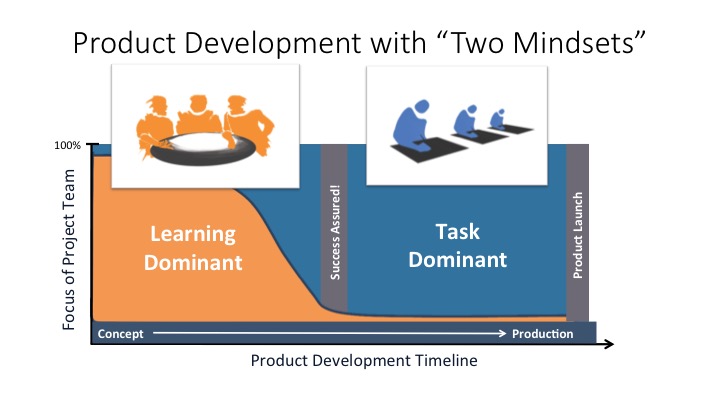
Agile Design Controls Part 2 Webinar: Dynamic Risk Management for SW-Intensive Medical Devices
This webinar describes a dynamic approach to managing risk analysis and risk controls to keep up with fast moving product development teams. Learn the nuts and bolts of how to integrate risk management into the overall product development workflow using a requirements management SW tool to create a more flexible and rigorous approach than standalone […]