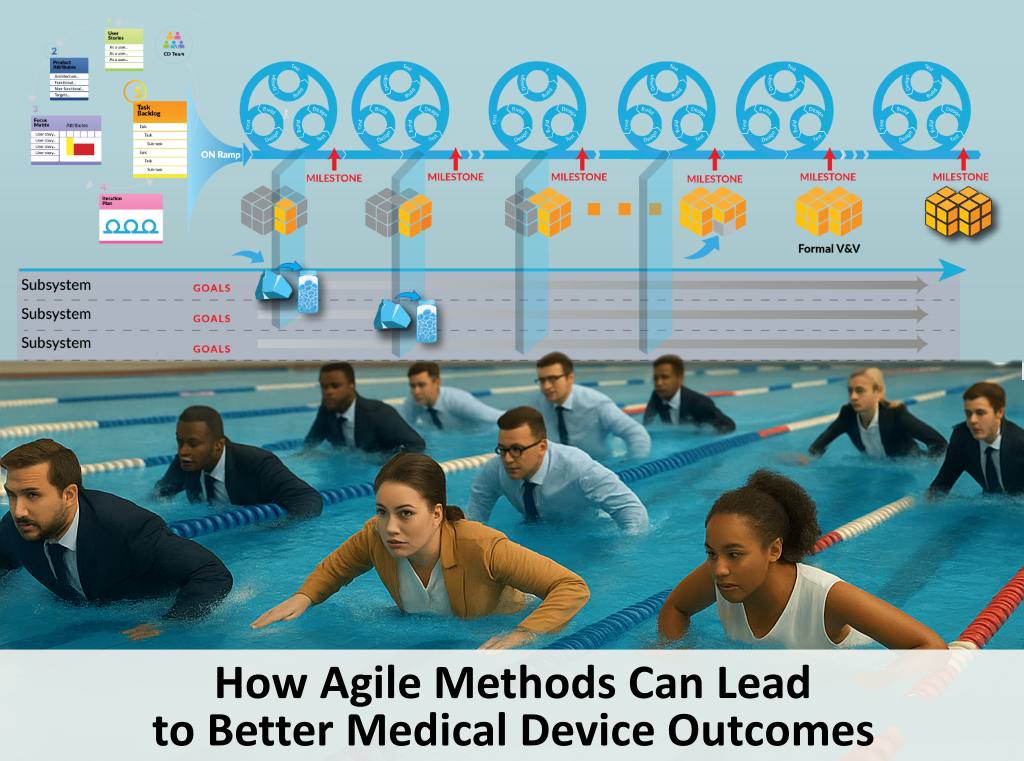
Secrets to Unlocking Agile Benefits for Medical Device Development
Agile medical device development can provide tremendous benefits but agile methods must be modified for successful hardware development and regulatory compliance. The MAHD Framework provides a proven process with these modifications.
Secrets to Unlocking Agile Benefits for Medical Device Development Read More »