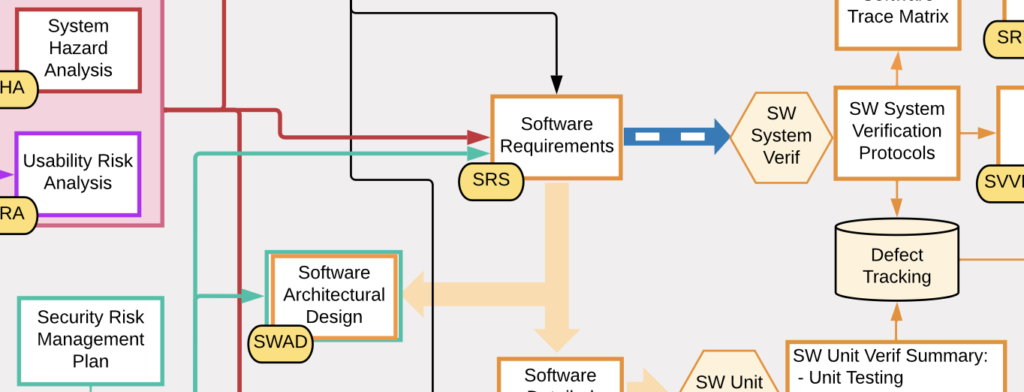
SaMD Documentation Map
This SaMD Documentation Map illustrates the formal documentation needed for a typical Software as a Medical Device (SaMD) product. The map is a graphical representation of the interdependencies of the product documentation and what is needed for an FDA 510(k) submission.
SaMD Documentation Map Read More »