The proliferation of software in modern medical devices has enabled innovative new applications and many benefits to patients. However, it has also created greater challenges for medical device companies: namely, managing greater product complexity and more reliance on third party software components. For connected devices there are the added challenges of cybersecurity risks, protecting patient data, and maintaining interoperability. All of this means that medical device companies must be adept at managing change, especially after product launch.
Let’s see what this looks like for an example connected medical device. The figure below shows a wearable heart monitor (smart watch) which communicates via BlueTooth with a mobile device which provides controls and feedback to the user and transmits patient data to the cloud where it is analyzed and stored. A cardiologist can then view the patient’s data and make decisions about whether to adjust medications or bring the patient in for additional exams.
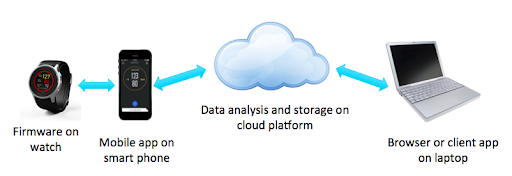
This distributed Internet of Things (IOT) architecture is very flexible and powerful but presents some key challenges for developing and sustaining products. Multiple parts need to work together to provide the medical benefit. That means that changes to one part, such as the mobile app, can trigger changes to other parts (the watch or software in the cloud). Each part has different software development methods and tools, which need to be managed differently, and include third party software components which can change independently from your product.
The changes needed after product launch for a hardware-only medical device, assuming it was optimally designed, are basically the following: changes to packaging and labeling, changes to the supply chain, and refinements to manufacturing processes. However, if the device contains software, there are many more potential changes after product launch as shown in the table below. Importantly, some of these changes, such as security vulnerabilities, are externally driven and cannot be planned and scheduled–these types of change force a medical device manufacturer to respond quickly to maintain a safe and effective product.
| Hardware-Only Devices | Hardware-Software Devices |
| • Changes to packaging / labeling • Changes to component suppliers • Refinements to mfg process steps | • Changes to packaging / labeling • Changes to component suppliers • Refinements to mfg process steps • SW changes due to updated OS or 3rd party library • SW changes to maintain interoperability • SW changes to address security vulnerability • Changes to GUI or other functions • Additional features |
Embracing change means setting up your organization, processes, and tools to efficiently manage changes during product development and after product launch. At the organizational level, your company needs clearly defined responsibilities for sustaining engineering and close collaboration with new product development. This ensures a continuum of product knowledge across the entire product life cycle. Companies that have a vaguely defined hand-off from product development teams to sustaining engineering or worse, have outsourced their software development, risk losing key product knowledge needed to maintain the product after launch.
What processes and tools are needed? The list below describes characteristics of organizations that are able to manage product change efficiently for software-intensive and connected medical devices:
- Agile methods for product development
- Design iterations are expected during development (and after product launch)
- Design controls are not forced to be sequential (don’t create a “waterfall” framework)
- Multiple iterations of risk management, design inputs, design outputs, V&V testing
- Scalable, flexible quality system processes for controlling design changes (not a one-size-fits-all approach)
- Well-defined software configuration and release process (“turn the crank”)
- Product designed for upgradeability (secure remote software patching, modular architecture, etc.)
- Good knowledge management systems to prevent loss of product knowledge over time
- Centralized issue tracking (not compartmentalized) – able to coordinate and synchronize changes to multiple hardware and software components in a distributed architecture
- Test automation – able to quickly execute a suite of automated software regression tests for every change to the software
- Streamlined compliance
- Minimize delays in review, approval, and release of records
- Eliminate redundant records
- Documentation automation – much of the required compliance documentation is generated automatically by the same software tools used for managing the product design and testing
Your company does not need to have all of these characteristics to be successful, but every one of them will help lower the cost of design changes. Most importantly, everyone needs to view medical device software as something that needs to evolve over time to maintain a high quality product. Becoming better and better at managing product design changes will enable your company to be both faster at product development and better at post-market maintenance, which translates into longer product life.


Pingback: Accelerating Value Delivery for Medical Device Software - Sunstone Pilot, Inc.