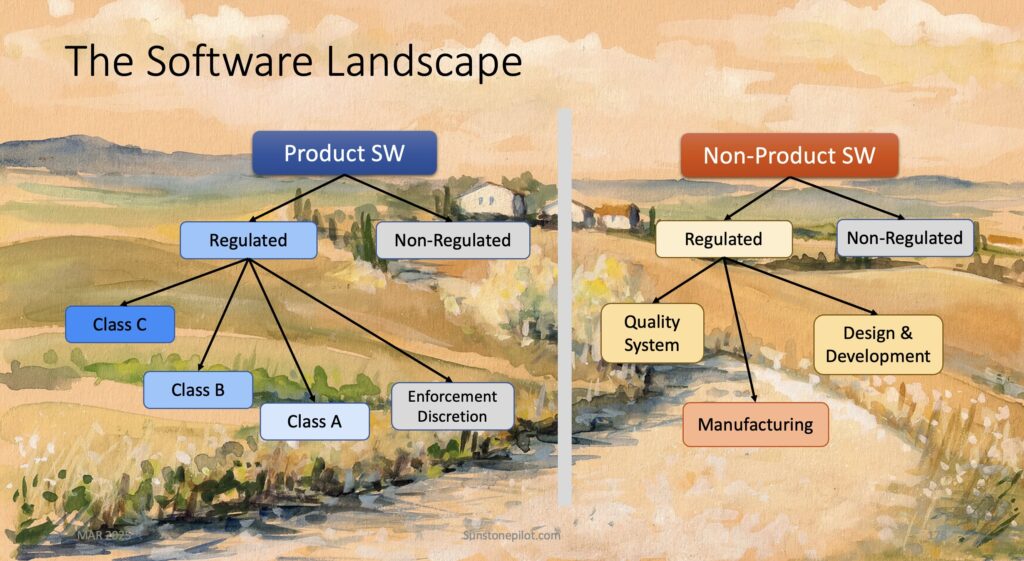
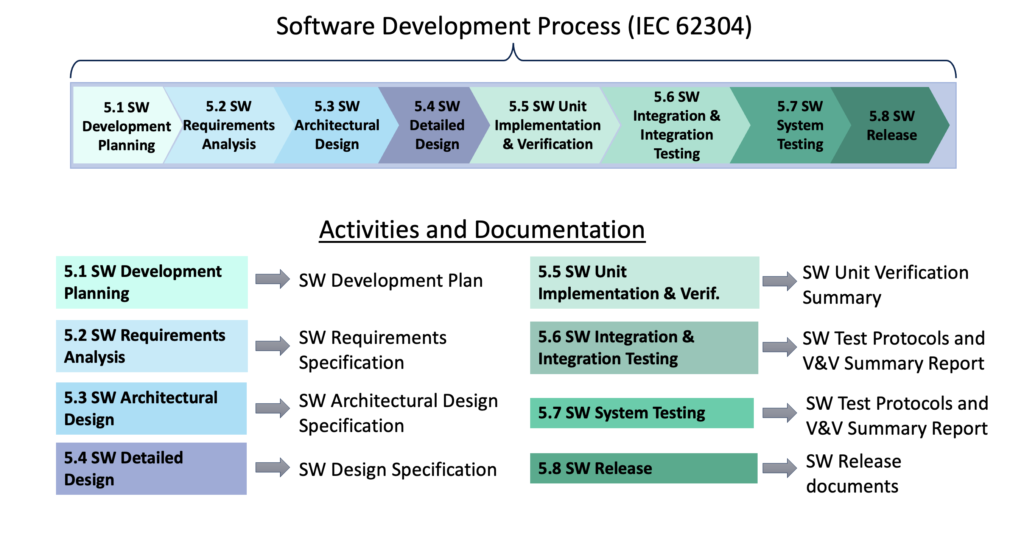
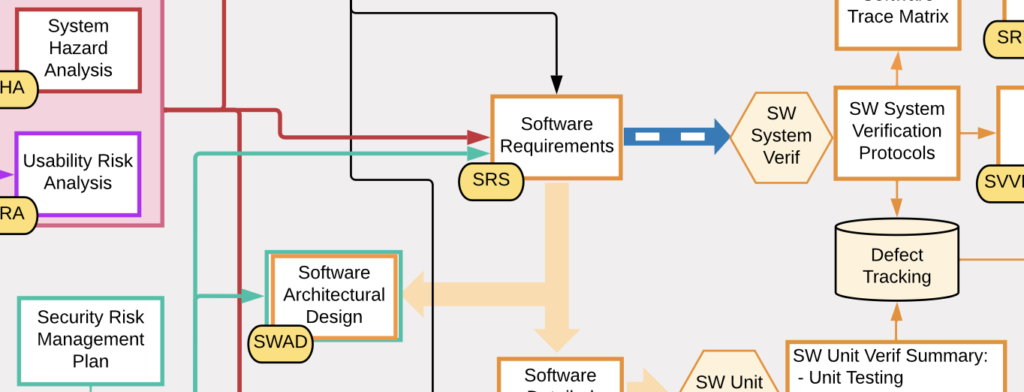
Understanding the Software Landscape
Understanding all of the types of software at a medical device company is crucial to knowing what is required for validating, documenting, and controlling each type
Understanding the Software Landscape Read More »