FDA Software Guidances and the IEC 62304 Software Standard
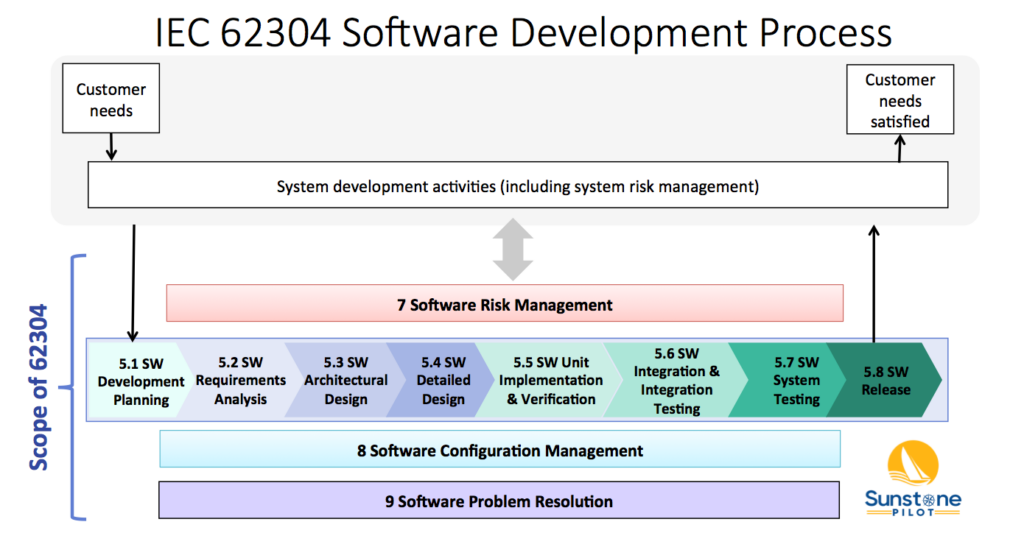
[updated NOV 2023] “One approach to satisfy two sets of rules” for medical device software As stated in the last blog post, there are two sets of rules for medical device software—twice the rules, twice the confusion. My recommendation is to base your software development procedures on the international IEC 62304 standard, which is easier […]
FDA Software Guidances and the IEC 62304 Software Standard Read More »