Buyer’s Guide for Requirements Management Tools for Medical Devices
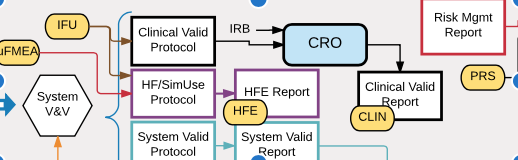
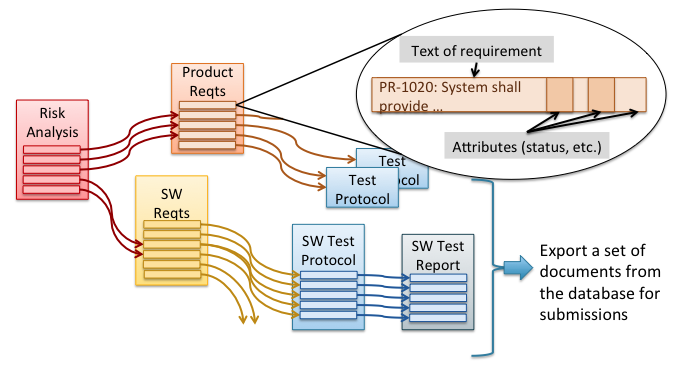
Maintaining traceability and DHF documentation with tedious manual methods burdens your engineers and risks compliance problems. Many medical device companies are now implementing requirements management (RM) software tools to automate traceability and DHF documentation. These tools can keep all of your risk management, requirements, and V&V documentation up to date while supporting rapid design iterations […]
Buyer’s Guide for Requirements Management Tools for Medical Devices Read More »