Agile Design Controls: How to Support Rapid Design Iterations for SW-Intensive Medical Devices
How to examples using Matrix ALM and JIRA Traditional methods for medical device development cannot keep up with the challenges of modern, software-intensive medical devices, which need to be developed iteratively and to be regularly updated after launch. Software tools for requirements management, test management, and defect tracking allow product development teams to make rapid
Newsletter V. 2018 Issue 3
My Notes on CyberSecurity I learned a lot about the latest trends in cybersecurity and how to protect medical devices and hospitals at the Connected Devices: CyberSecurity & Compliance conference this week hosted in San Francisco. Upcoming Events Agile Design Controls: How to Support Rapid Design Iterations for Software-Intensive Medical Devices In this webinar I’ll
Newsletter V. 2018 Issue 3 Read More »
Connected Devices: Cybersecurity & Compliance Summit
Is your medical device secure? I learned a lot about the latest trends in cybersecurity and how to protect medical devices and hospitals at a conference this week hosted in San Francisco and featuring presentations by cybersecurity experts from the medical device industry, from hospital systems, and from vendors of security tools. Medical device cybersecurity
Connected Devices: Cybersecurity & Compliance Summit Read More »
Newsletter V. 2018 Issue 2
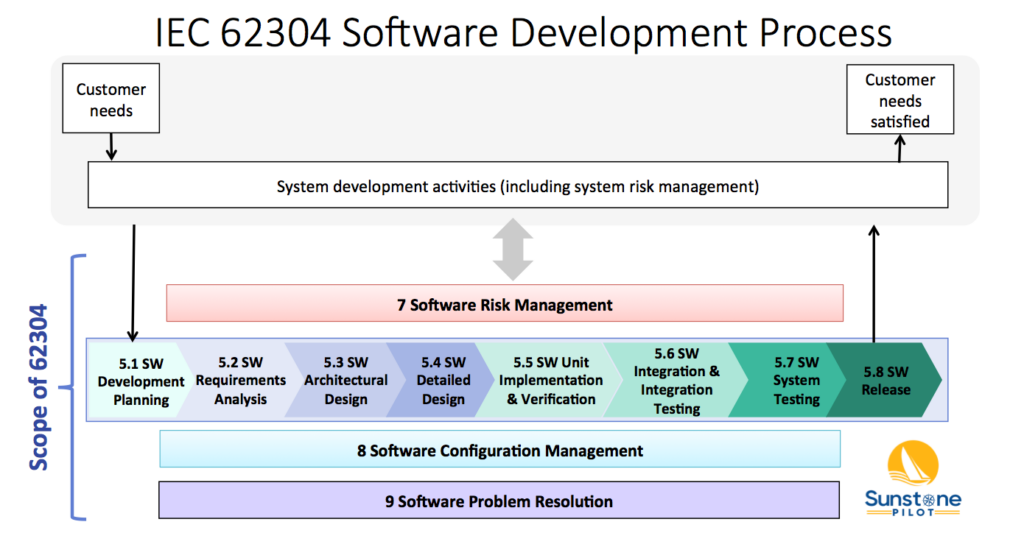
FDA Software Guidances and the IEC 62304 Software Standard There are two sets of rules for SW regulation—twice the rules, twice the confusion. My recommendation is to base your software development procedures on the IEC 62304 Standard and then include any additional adjustments needed to meet all the FDA requirements. This article details exactly what
Newsletter V. 2018 Issue 2 Read More »
FDA Software Guidances and the IEC 62304 Software Standard
[updated NOV 2023] “One approach to satisfy two sets of rules” for medical device software As stated in the last blog post, there are two sets of rules for medical device software—twice the rules, twice the confusion. My recommendation is to base your software development procedures on the international IEC 62304 standard, which is easier
FDA Software Guidances and the IEC 62304 Software Standard Read More »
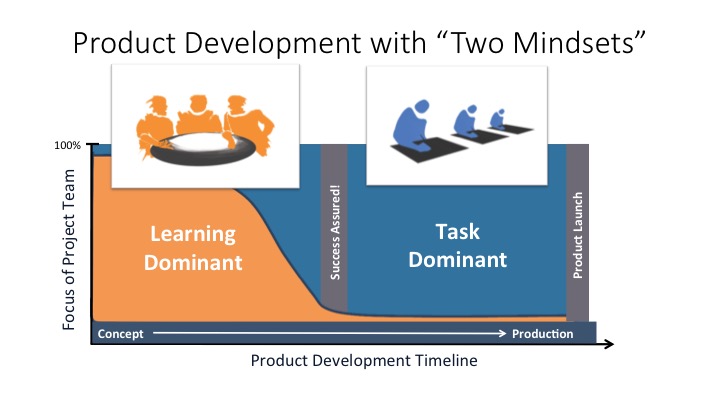
The Two Minds for Successful Product Development
Aaron Joseph and Roger Tang, PhD, Principal at HR Tang Consulting A successful product development process for innovative products is naturally comprised of two mindsets. Early in the product development process, where the uncertainty is the highest, the team’s focus is on rapid learning to close knowledge gaps. These knowledge gaps are those that are
The Two Minds for Successful Product Development Read More »
Newsletter V. 2018 Issue 1
Beginner’s Guide to Medical Device Software “Medical Device Software for Newbies” or “Why is this stuff so confusing?” This article is designed for people who are new to medical device software—either experienced with software but not medical device regulations or experienced with medical device hardware only. Why is software special? References on medical cybersecurity for
Newsletter V. 2018 Issue 1 Read More »
Intro to Medical Device Software
Beginner’s Guide to Medical Device Software “Medical Device Software for Newbies” or “Why is this stuff so confusing?” Many times I’m asked to explain to engineers how to manage compliance for medical device software development. From newcomers to experienced professionals, it remains a source of confusion and frustration. And a lot of the confusion comes
Intro to Medical Device Software Read More »
On-time and under budget
Aaron Joseph helped Luma Therapeutics develop our quality system procedures and DHF documentation for the Luma Light System in 2017. This was in preparation for an FDA 510k filing. He came in on time and under budget for his services and was a valuable addition to the team. He has a pleasant, relaxed demeanor, always
On-time and under budget Read More »