Intro to Medical Device Software
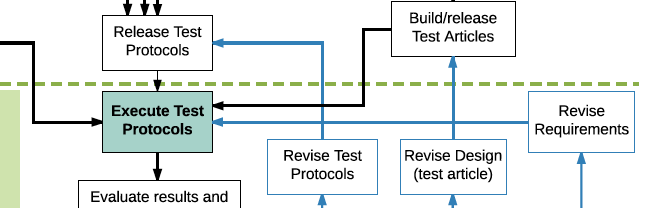
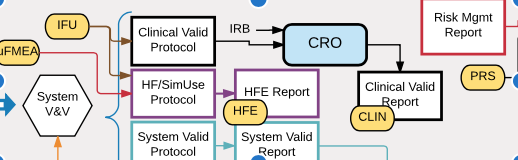
Beginner’s Guide to Medical Device Software “Medical Device Software for Newbies” or “Why is this stuff so confusing?” Many times I’m asked to explain to engineers how to manage compliance for medical device software development. From newcomers to experienced professionals, it remains a source of confusion and frustration. And a lot of the confusion comes […]
Intro to Medical Device Software Read More »