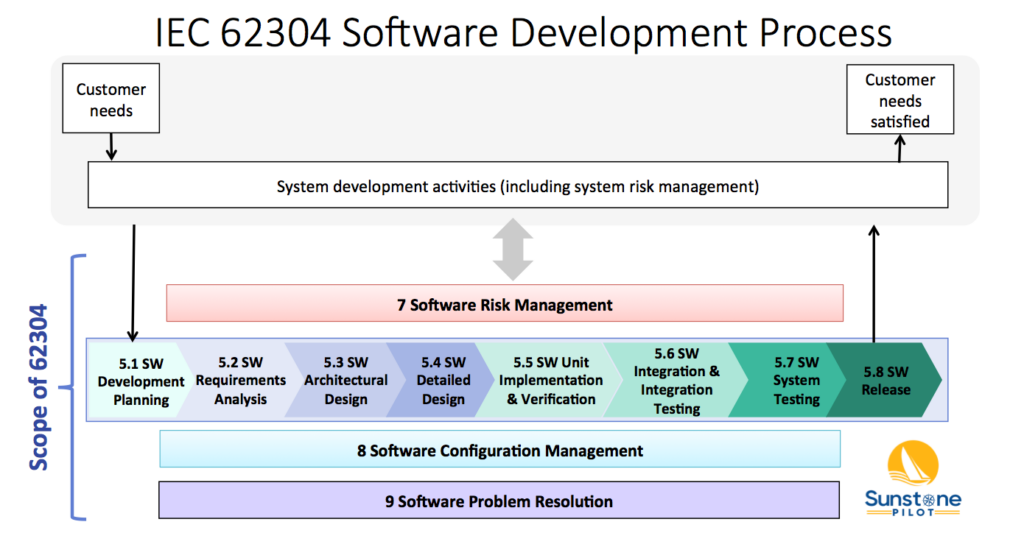
Software Testing Is Not Hardware Testing
Best practices for medical device HW testing are not best for SW testing Medical device development teams often ask me for help with software V&V. Even though they have their hardware development under control, these teams keep encountering software testing problems and the corresponding compliance documentation. Frequently, it’s because their company’s quality system, explicitly or […]
Software Testing Is Not Hardware Testing Read More »