Newsletter V. 2020 Issue 2
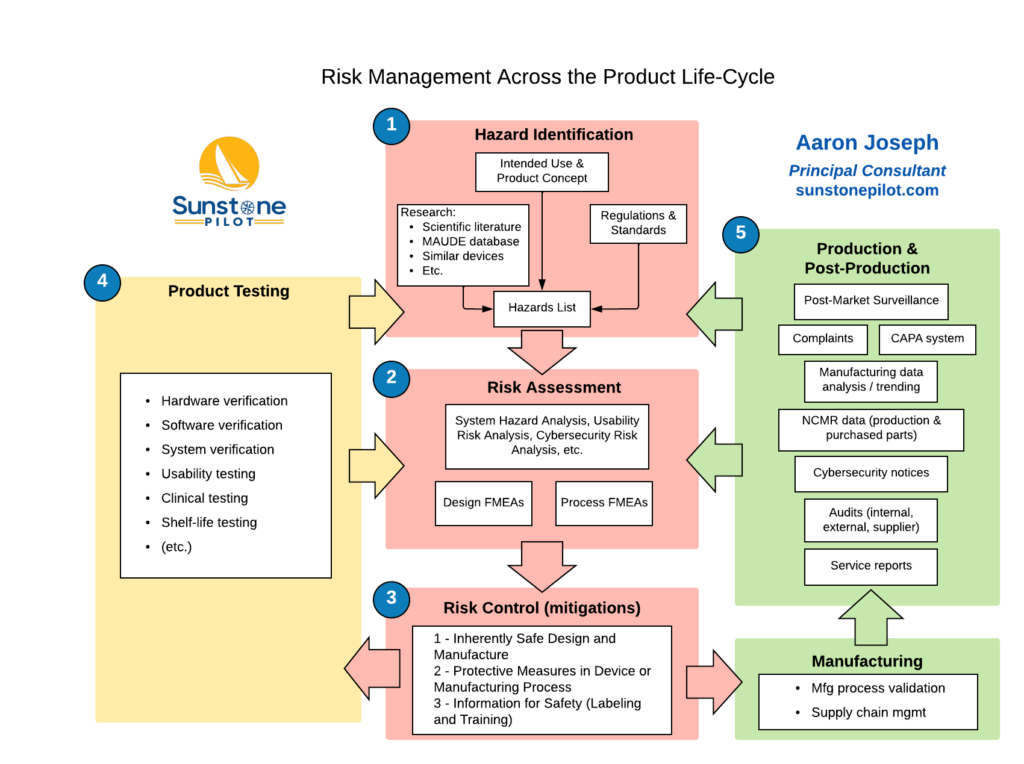
OCT 2020 Newsletter – Medical software challenges and new digital opportunities Some of the most promising innovations in healthcare technology today are based on software development and the digital health sector itself is a large and growing part of the industry (see below). Software enables many new opportunities in healthcare but also presents new challenges […]
Newsletter V. 2020 Issue 2 Read More »